Abstract
There are major progress in the upfront and relapsed treatments of multiple myeloma (MM) patients with the immunomodulatory agents, proteasome inhibitors, anti-CD38 monoclonal antibody therapy. In this context of availability of multiple therapeutical options, admission policies and the management of MM patients with life-threatening events who require intensive care need to be reconsidered. In fact, there are few studies that evaluate these questions for MM patients treated with modern therapies. The objective of this retrospective study is to determine prognostic factors of MM patients admitted in intensive care unit (ICU) and evaluate their outcomes.
Between June 2014 and December 2019, 72 MM patients were admitted in ICU of teaching hospital of Lyon, France ; on 620 patients with MM (12%). Medical history with clinical, biological and treatment data concerning MM were abstracted from medical records. We also collected medical variables related to intensive cares especially the causes of ICU admission, Sequential Organe Failure Assessment (SOFA) and Simplified Acute Physiology Score 2 (SAPS 2) and the management and events in ICU. Prognostic factors associated with 90-day mortality were investigated in univariate and multivariate analyses using Cox model.
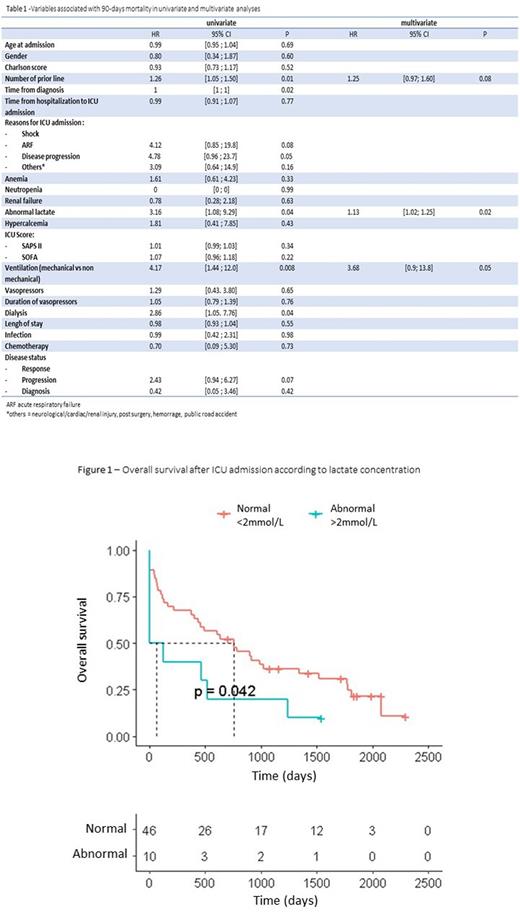
The median age of MM patients at the ICU admission was 67 years (62-74). The majority of patients were previously exposed to immunomodulatory agents (n=52, 72%), proteasome inhibitors (n=54, 75%), 19 (26%) received anti-CD38 monoclonal antibody and 27 (38%) received autologous stem cell transplantation (ASCT). The median of prior lines was 2 (1-3). At ICU admission, 29 (40%) patients were responders to therapy (complete remission/very good partial response/partial response) and 43 (60%) had progressive disease including patients at diagnosis (n=10, 14%). The median of days of ICU admission since the first day of hospitalization in conventional unit was 1 (0-2). The cause of ICU admission were as follows: shock (n=18, 25%), acute respiratory failure (n=18, 25%), disease progression (n=13, 18%), neurologic injury (n=6, 8%), cardiac injury (n=5, 7%), kidney injury (n=4, 6%), post surgery (n=4, 6%), hemorrage (n=3, 4%), public road accident (n=1 ; 1%). Twenty-three patients (32%) benefited from multiple ICU admission : 16 two stays, 4 three stays, 3 more than four stays. Mechanical ventilation was required for 24 patients (33%), with a median duration of 7 days (2-11), 4 patients (6%) had non-invasive ventilation, 4 (6%) had high-flow nasal oxygene and25 (35%) had nasal oxygene. Vasoactive drugs was required for 12 patients during a median of 3 days (2-6) and renal remplacement therapy was used in 9 patients. The median lengh of ICU stays was 5 days (2-8). In total, 15 patients (21%) died during ICU stays of differents causes : 8 (54%) of infection, 5 (33%) of disease progression, 2 (13%) of other cause. Fifty-two (72%) patients were put on anti-infectious therapies. The main clinical infections was pulmonary (50%). Documented infections was found in 29 patients (56%). The most common germs were Gram-negative bacteria (48%) and then virus (21%). Fifty-two (72%) patients were able to have subsequent MM therapies after ICU admission, with a median number of lines of 1 (0-2). The 90-days and one year OS rates since ICU admission (first stay) were 69% and 58%, respectively. In univariate analysis integrating variables at day 1 and before ICU admission, number of previous lines for MM treatment, time between MM diagnostic and ICU admission and lactate concentration were associated with 90-day mortality. In multivariate analysis, only lactate concentration (HR=1.13; 95%CI, 1.02-1.25; p=0.02) was associated with 90-day mortality (Table 1 and Figure 1). In a second multivariate analysis integrating also variables related to ICU management, only mechanical ventilation is associated with 90-day mortality (HR=3.68; 95%CI, 0.98-13.8; p=0.05).
This retrospective study showed that the rate of mortality in ICU for MM patients treated with modern therapy is closed to general population (20%) in favor of a broad ICU admission policies for these patients. Lactate concentration was the unique independant variable of 90-day mortality which is in aggrement with findings observe in ICU general population. These data are in favour of a closed collaboration between hematologists and intensivists for an optimal management of MM patients with a life threatening events.
Disclosures
Karlin:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial Support travel & scientific meetings; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial Support travel & scientific meetings; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Sesques:Chugai, Novartis, and Kite/Gilead: Research Funding. Salles:Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys AG, Amgen, Bayer, Epizyme, Regeneron, Kite, a Gilead Company: Honoraria; AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, a Gilead Company, Miltenyi, MorphoSys, Takeda, and VelosBio: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys AG, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol-Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, Kite, a Gilead Company, Loxo, Rapt: Consultancy. Bachy:Kite, Gilead, Novartis, Roche, Incyte, Miltenyi Biotech, Takeda, Sanofi: Honoraria; Roche, Gilead, ADC Therapeutics, Takeda, Novartis, Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen, BMS: Research Funding; Hospices Civils de Lyon: Current Employment. Ghesquieres:Abbvie: Honoraria; Gilead: Consultancy, Honoraria; BMS: Honoraria; Roche: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal